Asaf Danziger, CEO (left)
William Doyle, Chairman
the business
of Novocure
In the early 2000s, Professor Yoram Palti, our founder and professor emeritus of physiology and biophysics at the Technion — Israel Institute of Technology, sought to leverage his expertise in biophysics to develop a new way to treat cancer that would destroy tumor cells while sparing healthy tissue and avoiding the life-altering side effects of existing cancer therapies.
Professor Palti founded Novocure™ to provide patients with a new cancer treatment based on his hypothesis, since proven, that low-intensity, alternating electric fields, when applied at specific frequencies, can disrupt cancer cell division and cause cancer cell death. This innovative treatment — Tumor Treating Fields, or TTFields — has become a completely new approach to cancer therapy.
When we started, it seemed incredible to many that electric fields could be tuned to kill cancer cells without inducing the systemic side effects so common with cancer therapies currently in use. But the strength of our data and the growing body of patient stories now speak for themselves.
Establishing, building and growing an innovative company to provide patients with a new cancer treatment modality demands determination, perseverence and an unwavering focus on the scientific evidence to keep moving forward regardless of the obstacles or resistance that may be encountered. This is how we operate.
We are dedicated to our mission of making TTFields available to all cancer patients who may benefit from it.
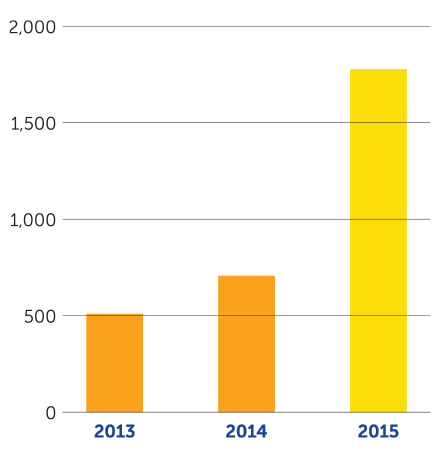
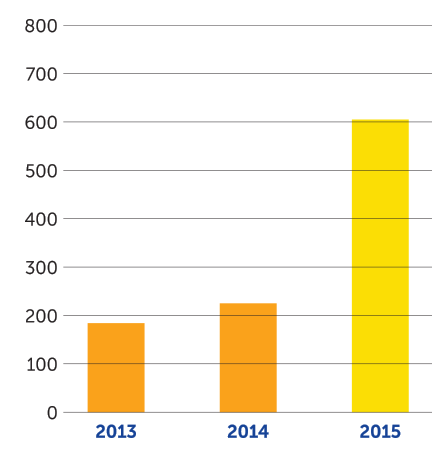
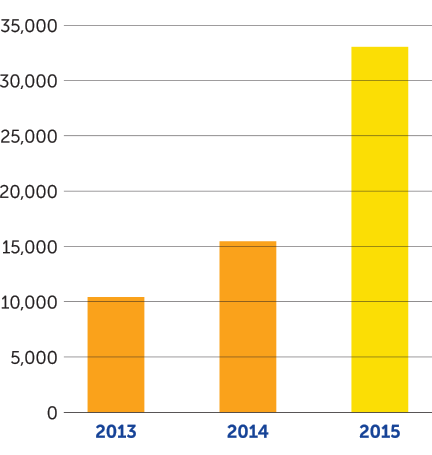
In the fourth quarter of 2015, we achieved several key milestones supporting our mission. On October 5, 2015, we received U.S. Food and Drug Administration approval of Optune — a portable, noninvasive device that delivers TTFields therapy — together with temozolomide for newly diagnosed glioblastoma (GBM). On December 15, 2015, the results of our EF-14 phase 3 pivotal trial of Optune together with temozolomide were published in the Journal of the American Medical Association with the conclusion that adding TTFields therapy to maintenance temozolomide chemotherapy significantly prolonged progression-free and overall survival in newly diagnosed GBM. As of December 31, 2015, we had 605 active patients on Optune therapy compared to 225 at the end of 2014, an increase of 169 percent. We continue to see growth in the number of active patients in the first quarter of 2016 and recently crossed the 700 active patient mark.
The strength of our data and the growing body of patient stories now speak for themselves.
We also continue to improve our treatment for GBM. Our second generation Optune system, which is half the size and half the weight of our first generation Optune system, is available to all new patients in Europe. We have filed for regulatory approval with the FDA and, assuming we do not receive comments or requests for additional information, we hope to begin marketing our second generation Optune system in the United States after approval later this year.
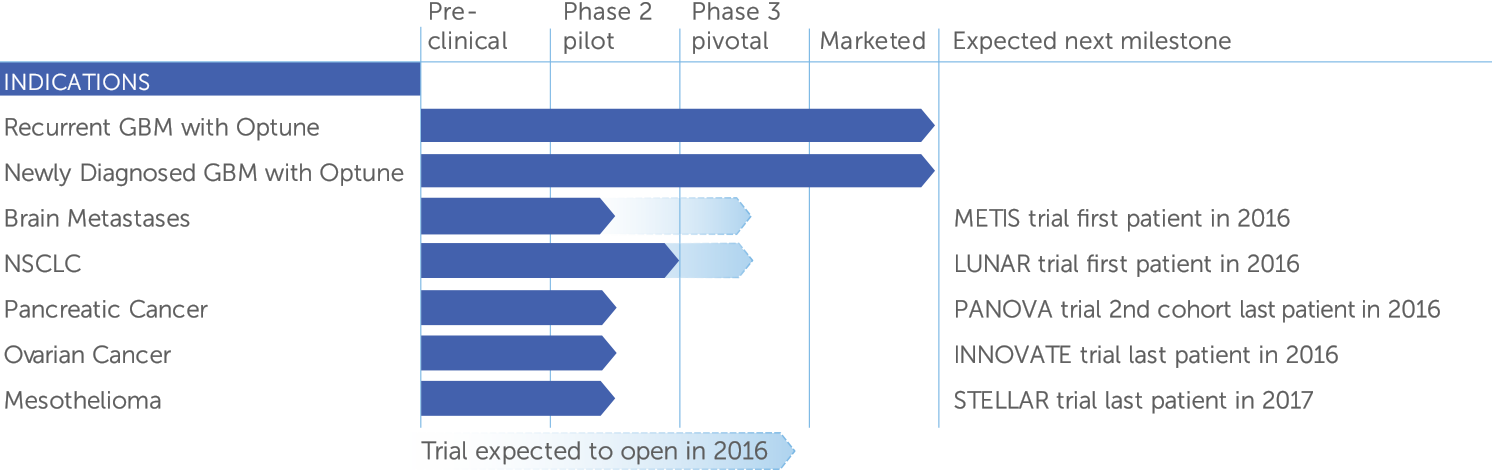
We are making progress toward treating other solid tumors in addition to GBM. We have five ongoing or completed phase 2 pilot trials in brain metastases, non-small cell lung cancer, ovarian cancer, pancreatic cancer and mesothelioma. Early in 2016, we presented data from the first cohort of our PANOVA phase 2 pilot trial in advanced pancreatic cancer demonstrating that TTFields may improve survival of patients with this terrible disease. Based on the positive data from the first cohort of PANOVA patients, we have accelerated our planning of a phase 3 pivotal trial in pancreatic cancer. We also expect to open phase 3 pivotal trials in brain metastases and non-small cell lung cancer in 2016.
Over a decade ago, we joined Novocure for the opportunity to make a meaningful difference in the lives of cancer patients. We reached a pivotal milestone in 2015 with the FDA approval of Optune for newly diagnosed GBM in the United States. Our passion to bring Optune to all GBM patients who may benefit from it and to develop TTFields for additional solid tumor cancers is greater than ever.